Human Tonsil Organoids to Characterise Germinal Center Dynamics

Mustafa Ghanizada, Immunologist, Department of Immunology and Microbiology, UCPH
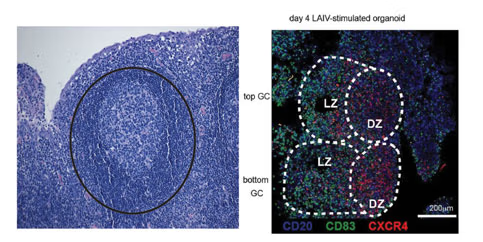
For decades, mice have been the preferred experimental animals for immunologists when developing experimental systems for diseases such as infections in humans. Inbred mice and mouse models have been extensively utilized in immunology research, providing insights, mechanistic understanding, and therapeutic advancements such as monoclonal antibodies, Tcell therapy, and checkpoint inhibitors. However, compared to mice, the human immune system exhibits greater complexity, dynamics, and interplay between host factors, proteins, cells, environment, and pathogens. Understanding and unraveling these human-specific aspects have become a pressing challenge in immunology. To address this challenge, the laboratory of Mark M. Davis at Stanford University has developed a unique in vitro system using human tonsil cells to form functional organoids capable of mounting adaptive immune responses to vaccines. Tonsils, secondary lymphoid tissues containing germinal centers (GCs), play a crucial role in facilitating interactions between follicular T helper (FTH) cells and B cells. This interaction is essential for generating high-affinity antibodies that confer protection. When human tonsil organoids are challenged with influenza or other vaccines, they demonstrate the ability to produce specific, high-affinity antibodies and generate appropriate T cell responses within a week to ten days of culture. The organoids exhibit the formation of germinal centers with the characteristic morphology and facilitate affinity maturation and class switching, which are key features of an adaptive immune response.
We propose integrating CRIPSR-cas9 gene-editing with the tonsil organoid to investigate the regulation of auto-reactive T and B cells during germinal center responses. Regulatory T cells can suppress auto-reactive T and B cells, but the precise mechanisms are unknown. These regulatory T cells can be genetically modified to remove functionality before organoid formation. These cultures can be analyzed to map the network of regulatory pathways that suppress human auto-reactive T and B cells. These results will give a revolutionary insight into key checkpoints that control immunity and pave the way for new treatments for autoimmune diseases and even cancer immunotherapy.