CNS Drug Delivery and Barrier Modelling

Mie Kristensen, Associate Professor, Department of Pharmacy, UCPH
Line Hagner Nielsen, Groupleader, Associate Professor, Department of Health Technology DTU
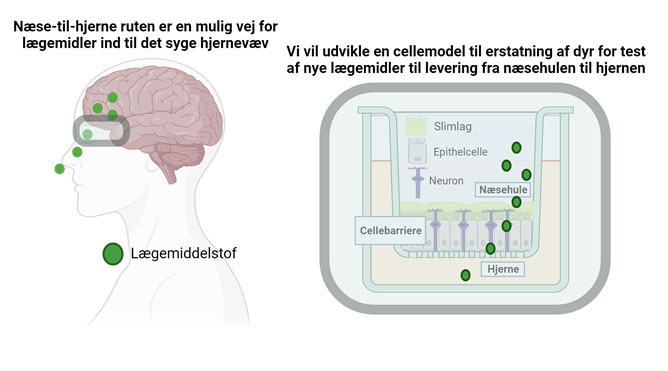
The number of people diagnosed with brain diseases is increasing. However, the development of novel medicine to treat these has not followed. Medicine administered via conventional injections do not pass from the blood stream to their site of action within the brain tissue due to the “blood-brain barrier”, the barrier properties of the brain blood vessels. Therefore, we need alternative ways to deliver otherwise potent medicine to the brain. The upper part of the nasal cavity represents an alternative route into the brain tissue; a route whereby the blood-brain barrier is bypassed – and medicine can be administered through a nasal spray instead of an injection.
The majority of studies in “nose-to-brain” delivery of medicine are performed in mice or rats. However, the nasal cavity anatomy of rodents is markedly different from humans; resulting in poor translation from the early pre-clinical studies to the clinical studies performed humans – and thus irrelevant use of animals. I addition to that, do animal experiments not reveal the mechanisms underlying transport of medicine across the olfactory mucosa, the barrier separating the nasal cavity from the brain. However, we lack representative cell culture models, which can provide us with such mechanistic understanding that is highly relevant for the development of novel medicine, which is able to pass through the olfactory mucosa. As example, does current models lack sensory neurons, which are believed to be central for the transport of medicine from the nasal cavity and into the brain.
With this project, we will develop a human-based cell culture model representing the nasal olfactory mucosa. We will do that by culturing the human nasal epithelial cell line RPMI2650 together with human stem cell-derived neurons in a Transwell® system, thereby serving as a model for the olfactory mucosa. We will use our model to investigate to which extent model-drug compounds permeates the olfactory mucosa, and thereby, with time, replace the pre-clinical animal studies prior human clinical trials. Our model will moreover enable us to perform studies revealing the cellular mechanism underlying transport of novel drug compounds across the nasal olfactory mucosa. Ultimately, contributing with knowledge supporting the development of novel medicine, which effectively reach their target site of action in the brain.