Skin cancer model without use of animals
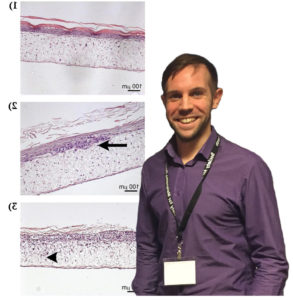
Malignant melanoma is the most deadly form of skin cancer, for which there are no consistently beneficial treatments. New therapeutic approaches for the treatment of melanoma focus on reducing tumour cell division and/or invasion, to slow tumour growth and prevent spread of the disease. Testing the safety and efficacy of new therapeutic strategies currently relies heavily on animal models, which may cause suffering and distress to the animals involved. Furthermore, mouse models aimed at studying the initial stages of melanoma development and invasion within mouse skin may not accurately represent human disease due to differences in the structure of skin between mice and humans. Therefore, to replace the use of mice for the investigation of local, early stage melanoma development, invasion and therapy response, I have recently developed and optimised an in vitro full-thickness melanoma skin equivalent model.
This novel assay accurately recreates the pathological progression of melanoma cells within their original human skin microenvironment as they divide and invade through the basement membrane, resulting in the breakdown of Type VII collagen. The aims of the current project are to modify the current protocol for constructing melanoma skin equivalents to eliminate the use for animal derived components such as bovine pituitary extract; to develop a nucleotide aptamer alternative to animal-derived antibody detection of Type VII collagen; and use the developed aptamer to validate the melanoma skin equivalent constructed with animal component free media by comparing breakdown of Type VII collagen with that observed in melanoma skin equivalents from a previous project created with media containing animal components. The current skin equivalent model is currently used within our lab as well as other labs for a wide range of applications; therefore adaptation of this model to an animal-component free methodology will ensure future studies using this skin equivalent are truly animal free.








